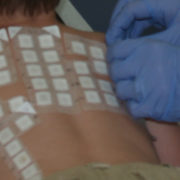
T.R.U.E. Test (patch test) receives pediatric indication
T.R.U.E Pediatric indication: T.R.U.E. Test (patch test) receives approval letter from Food and Drug Administration (FDA) for children 6 and older. After 12 years of investigations, evidence and data indicating that the patch testing is safe and efficacious in children suffering from recalcitrant dermatitis – the FDA has approved T.R.U.E Pediatric indication – the use of TRUE test to aid in the diagnosis of ACD in children.
This is the first commercially available patch test to receive approval for use in children 6 and older. The T.R.U.E. test has 36 components, one is a negative control.
To read more about patch testing in children:
Article on Pediatric Allergic Contact Dermatitis.
“August 25, 2017
Dear Ms. Sullivan:
SUPPLEMENT APPROVAL PMR FULFILLED
August 25, 2017
We have approved your request dated October 26, 2016, to supplement your Biologics License Application submitted under section 351(a) of the Public Health Service Act (42 U.S.C. 262) for Thin-layer Rapid Use Epicutaneous Patch Test (T.R.U.E. TEST), to use as an aid in the diagnosis of allergic contact dermatitis in persons 6 years of age and older whose history suggests sensitivity to one or more of the 35 substances included on the T.R.U.E. TEST panels.”